
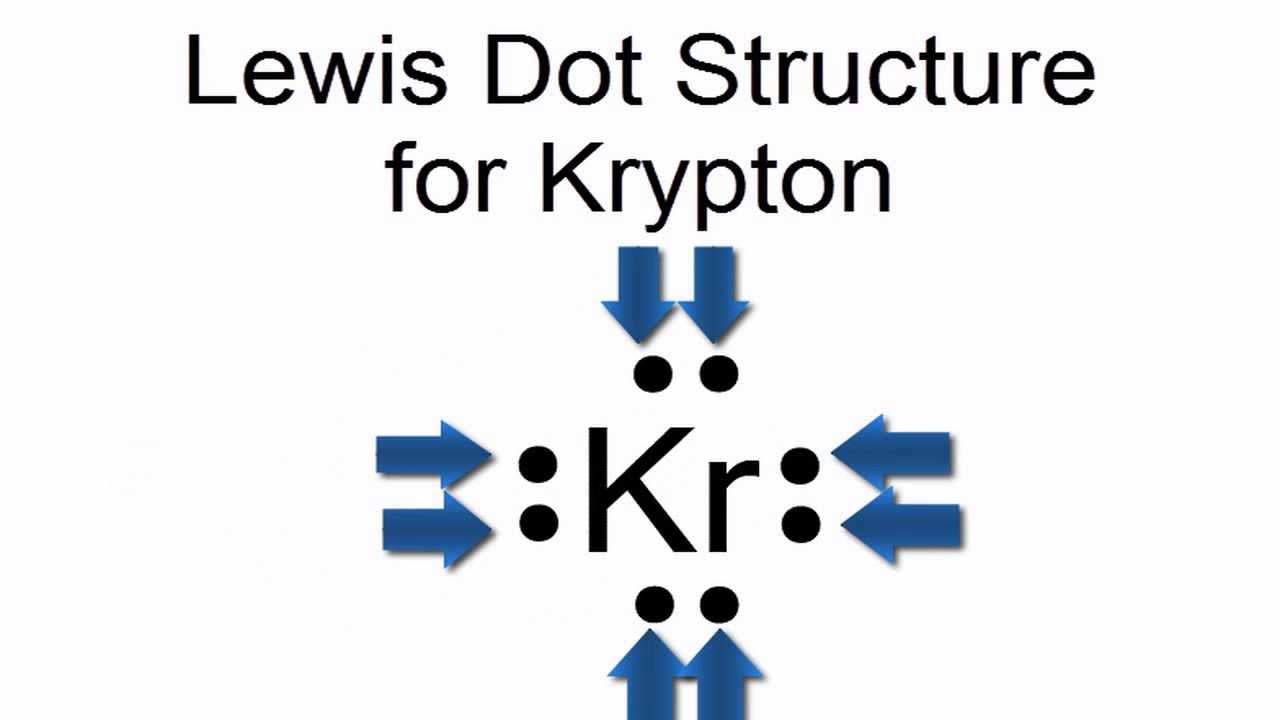

1s orbital present in the K orbit having minimum energy is filled first, with a maximum capacity of two electrons. 46) 46) The hybrid orbital set used by the central atom in KRF2 is B) sp2 mu D) sp³d E) sp3 aventlu C) sP A) sp³d2 o s odesiood s lislenosdt gana Dor vri om tt moonsnimo vis lo na nilo 08.n b H0. Because its boiling point (153.4 ☌, or 244. The electronic configuration of the krypton is expressed in the form of the diagram as given below-. Atomic Properties of Krypton Oxidation states: 2, 1, 0 Valence Electrons: 4s2 4p The ionization potential of an atom: 13.94 Ionization energies: 1st: 1350.8. Choose the one alternative that best completes the statement or answers the question.
#ELECTRON CONFIGURATION OF KRYPTON PLUS#
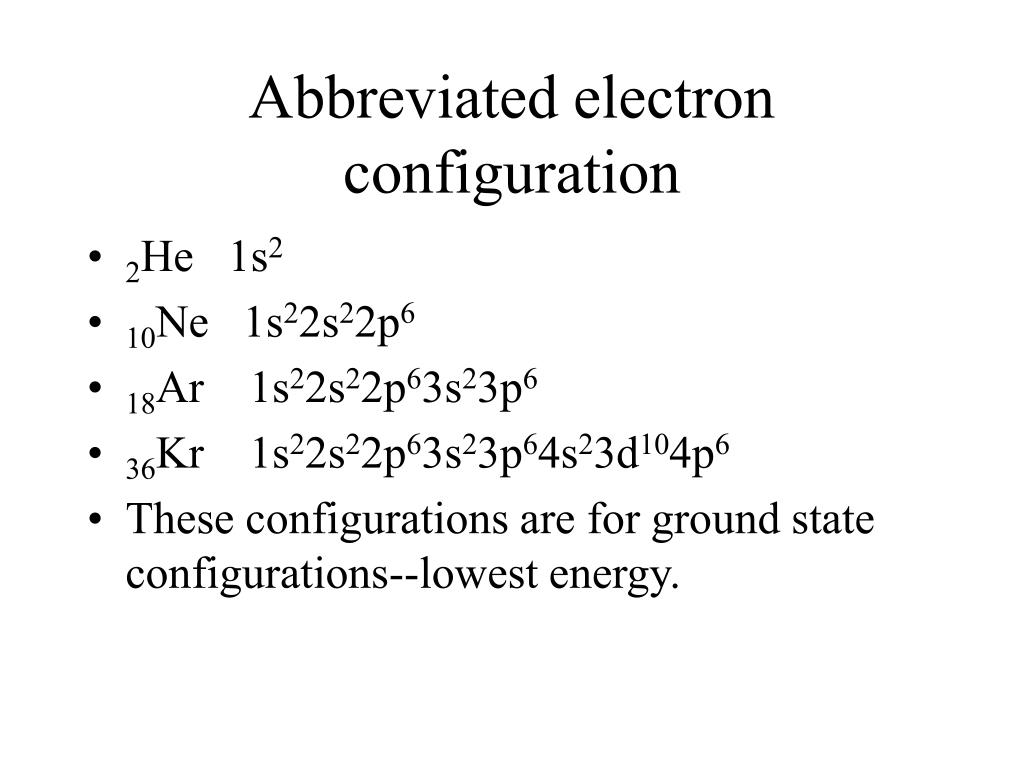
Thus, molybdenum has the electron configuration of krypton plus one electron in the 5s orbital. To obtain a +1 charge it loses an electron, resulting in a configuration of Ar. Molybdenum (Z 42) has an electron configuration Kr5s14d3. biiw ni 44) 44) As successive electrons are removed from an element, the ionization energy lqo od tada salg o bow alt h wA TO en so Jsshta don 45) Calculate the bond energy of C-F given that the heat of atomization of CHFCIBR is 1502 kJ/mol, and that the bond energies of C-H, C-Br, and C-Cl are 413, 276, and 328 kJ/mol, respectively. This page contains information for the element krypton in periodic table. Potassium (K) is orignially in the electron configuration of Ar4s1. And hence it does not have more chemical properties. As krypton is chemically stable, it does not react with any other elements. This indicates that it has a completely filled outer shell, which makes it chemically stable. Write the word or phrase that best completes each statement or answers the question. The electron shell configuration of krypton is 2, 8, 18, 8. Electron configuration, Ar 3d104s24p6, CAS number,. 43) 43) The condensed electron configuration of krypton, element 36, is A) 3d104s24p6 B) 4s23d8 C) 4s4 D) 4s43d8 E) (Ar]4s43d4 lo a dw ot of mils t boll mu oo beupil ogm obenbrurd lo bo SHORT ANSWER. Atomic number, 36, Relative atomic mass, 83.798.


 0 kommentar(er)
0 kommentar(er)
